44 what is an open label study
opensource.org › historyHistory of the OSI | Open Source Initiative The “open source” label was created at a strategy session held on February 3rd, 1998 in Palo Alto, California, shortly after the announcement of the release of the Netscape source code. The strategy session grew from a realization that the attention around the Netscape announcement had created an opportunity to educate and advocate for the ... en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered. [1]
pubmed.ncbi.nlm.nih.gov › 26338525FOLFOXIRI plus bevacizumab versus FOLFIRI plus ... - PubMed Methods: TRIBE was an open-label, multicentre, phase 3 randomised study of patients (aged 18-70 years with Eastern Cooperative Oncology Group [ECOG] performance status of 2 or less and aged 71-75 years with an ECOG performance status of 0) with unresectable metastatic colorectal cancer who were recruited from 34 Italian oncology units. Patients ...

What is an open label study
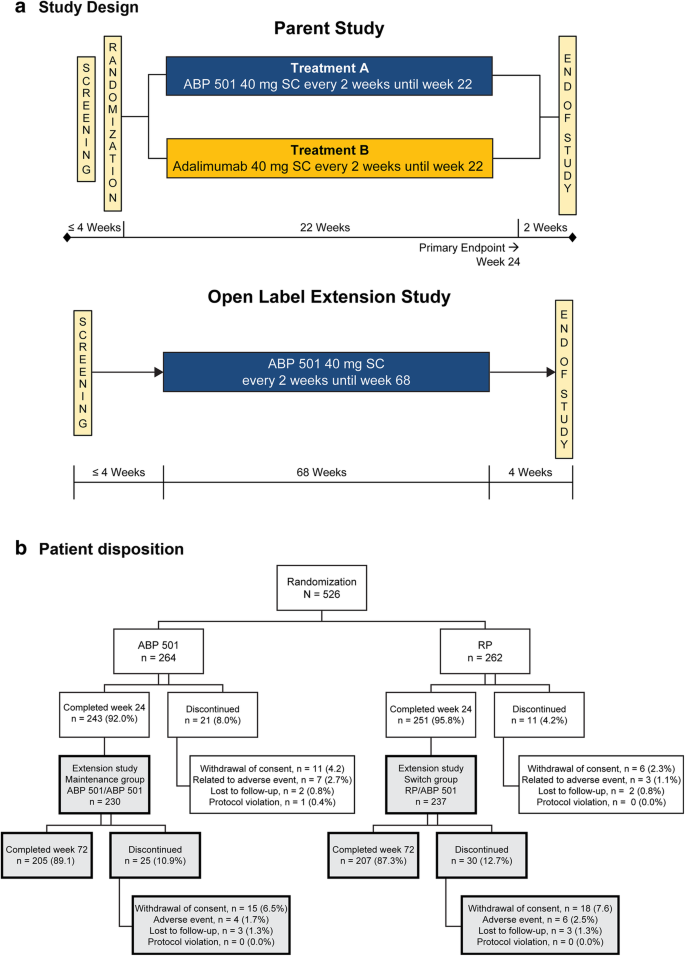
› journals › langasTrifluridine–tipiracil plus bevacizumab versus capecitabine ... Dec 02, 2022 · As this was an open-label study, investigators and patients were not masked to treatment allocation. Procedures Patients assigned to the trifluridine–tipiracil plus bevacizumab group received oral trifluridine–tipiracil 35 mg/m 2 twice a day on days 1–5 and 8–12 plus an intravenous infusion of bevacizumab 5 mg/kg on day 1 and day 15 of ... pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... However, this can only occur if the open-label extension study is designed, executed, analysed and reported competently. Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and ... › journals › lancetPembrolizumab alone or with chemotherapy versus cetuximab ... Oct 31, 2019 · The KEYNOTE-048 study was a randomised, open-label, phase 3 study done at 200 medical centres in 37 countries (appendix pp 2–5). Participants were eligible for enrolment if they were aged 18 years or older; had pathologically confirmed squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, or larynx that was recurrent or ...
What is an open label study. › health-topics › study-qualityStudy Quality Assessment Tools | NHLBI, NIH A study of the relationship between smoking and CVD events illustrates this point. Such a study needs to control for age, gender, and body weight; all are associated with smoking and CVD events. Well-done case-control studies control for multiple potential confounders. › journals › lancetPembrolizumab alone or with chemotherapy versus cetuximab ... Oct 31, 2019 · The KEYNOTE-048 study was a randomised, open-label, phase 3 study done at 200 medical centres in 37 countries (appendix pp 2–5). Participants were eligible for enrolment if they were aged 18 years or older; had pathologically confirmed squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, or larynx that was recurrent or ... pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... However, this can only occur if the open-label extension study is designed, executed, analysed and reported competently. Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and ... › journals › langasTrifluridine–tipiracil plus bevacizumab versus capecitabine ... Dec 02, 2022 · As this was an open-label study, investigators and patients were not masked to treatment allocation. Procedures Patients assigned to the trifluridine–tipiracil plus bevacizumab group received oral trifluridine–tipiracil 35 mg/m 2 twice a day on days 1–5 and 8–12 plus an intravenous infusion of bevacizumab 5 mg/kg on day 1 and day 15 of ...

























![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)







Komentar
Posting Komentar